The importance of obtaining FDA approval should not be under-estimated. It is a significant accomplishment and a legal requirement for any firm to sell medical devices or drugs in the US, the world’s biggest healthcare market.
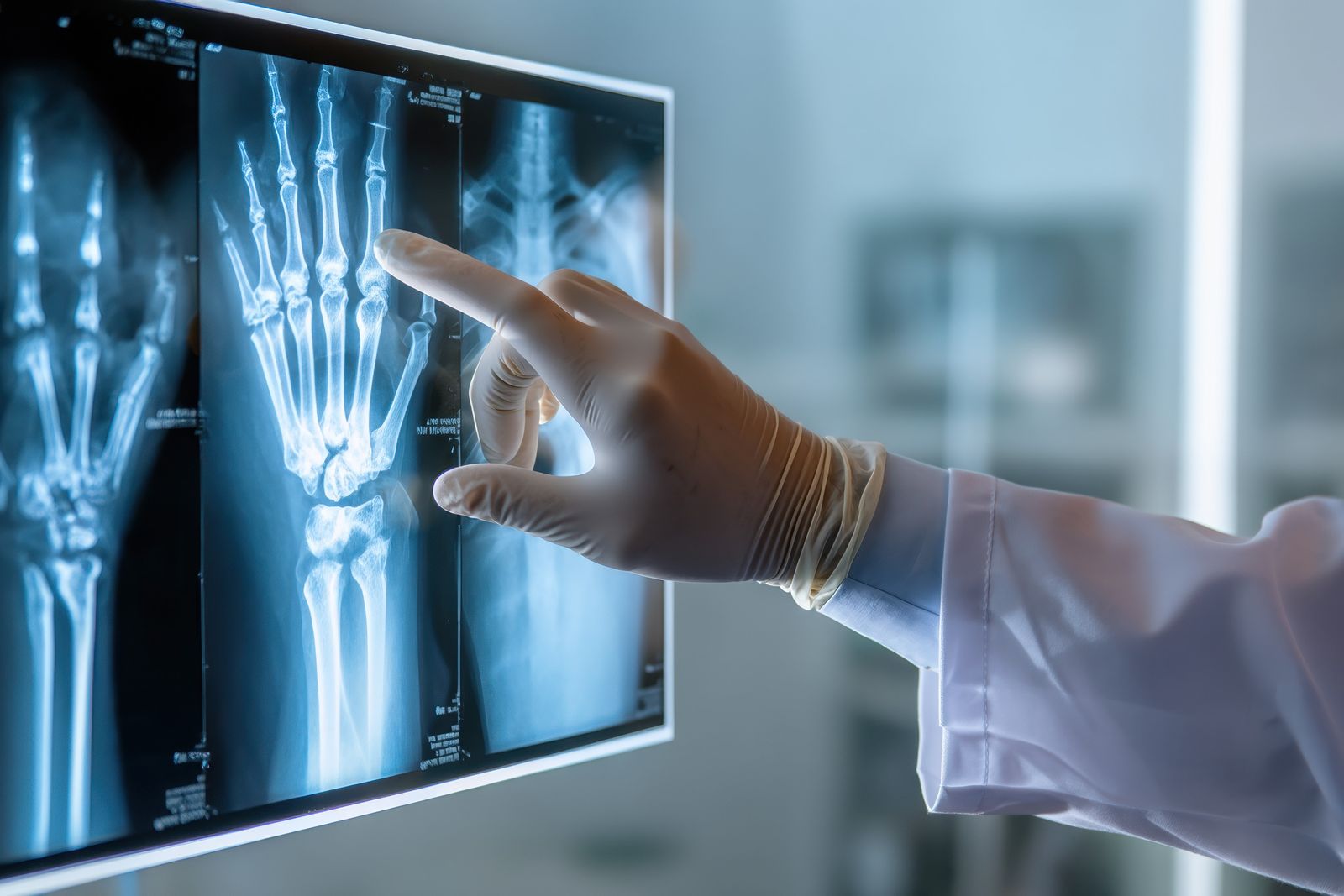
Today Avingtrans - a specialist engineer of mission critical components to the energy, data centre, medical and nuclear power sectors - announced that its subsidiary Adaptix Ltd had received FDA 510(K) clearance for its orthopaedic 3D image scanner, the Adaptix Ortho350.
This is an ultracompact low-dose, low-power imaging system that generates high quality 3D X-rays at the point-of-care to improve patient outcomes. Here it is not only designed for upper and lower extremities (eg hands, elbows, knees and feet) at a fraction of the radiation dose associated with traditional CT devices. But also ideal for scanning in local community facilities &/or doctor’s surgeries, and hence frees up scarce hospital resource.
Multiple UK & US distributors have already been appointed, whilst the Scottish manufacturing facility is also scaling up with production yields improving. Moreover Adaptix is selling the same technology to veterinary and industrial (eg material inspection) customers too, thus expanding the addressable market.
CEO Sarah Small of Adaptix Ltd commenting: “Securing FDA 510(k) clearance represents a major achievement for Adaptix and a pivotal moment in our mission to transform radiology. We are excited by the strong interest we have already received from healthcare providers and clinicians for our revolutionary orthopaedic imaging system. The Adaptix Ortho350 will enable ‘3D-First’ imaging in Primary Care, Intensive Care, and Emergency Departments across the US and beyond.”
Wrt the FY’25 numbers, Singer Capital Markets have a 600p/share target price (nb based solely on the engineering division), and are forecasting FY’26 turnover, EBITDA & adjusted EPS of £175m, £20.5m & 30.5p respectively. Additionally they believe the medical devices division is worth another £85m-£140m, or 251p-422p/share.
CEO Steve McQuillan of Avingtrans adding: “We are delighted with this important regulatory milestone. This clearance opens the door for entry into the substantial US orthopaedic imaging market and validates the commercial potential of Adaptix Ltd’s technology.”
Disclosure: Avingtrans is a Vox Markets client.