The clinical trial data keeps on getting better and better for Avacta’s (AVCT) flagship AVA6000 pro-drug, a novel form of generic chemotherapy drug doxorubicin. After initially increasing the dosage in February 2022 by 50% from 80mg/m2 to 120mg/m2, the company said today that it had lifted this to 160mg/m2, after the Safety Data Monitoring Committee (SDMC) recommended the move.
This appears to be a huge stamp of approval of AVA6000’s safety profile, since doxorubicin is one of the most powerful chemotherapies ever invented. Investors liked the news, sending the shares up 15%.
The big problem historically has been that in its raw form, the drug kills both cancerous and healthy tissues indiscriminately, especially around the heart. This means the normal approved maximum dose for conventional doxorubicin is between 60-75mg/m2 as a monotherapy.
Hence Avacta’s second dose escalation to a third patient cohort - i.e. using its chemo targeted fibroblast activation protein which is in high concentration in many solid tumours compared with healthy tissues - is more than double the usual permitted level and should lead to improved therapeutic effectiveness.
CEO Alastair Smith commented: "AVA6000 and the pre|CISION platform more broadly - have the potential to deliver safer and affordable oncology drugs that could significantly improve cancer patients' lives. We are very pleased with the progress being made with the study and look forward to seeing more data as it emerges from the trial."
Chief Development Officer Neil Bell adds: “The recommendation from the SDMC to initiate dosing in Cohort 3 with 160mg/m2 of AVA6000 is an endorsement of the emerging safety and tolerability profile in the patients enrolled in this study to date."
If ultimately successful, this could open up an enormous pipeline of next-generation pre|CISION pro-drug chemotherapies with significant clinical and commercial potential in a market that is expected to reach a total addressable size in excess of $74bn by 2027. Within this anthracyclines such as doxorubicin are forecast to grow to $1.38bn by 2024.
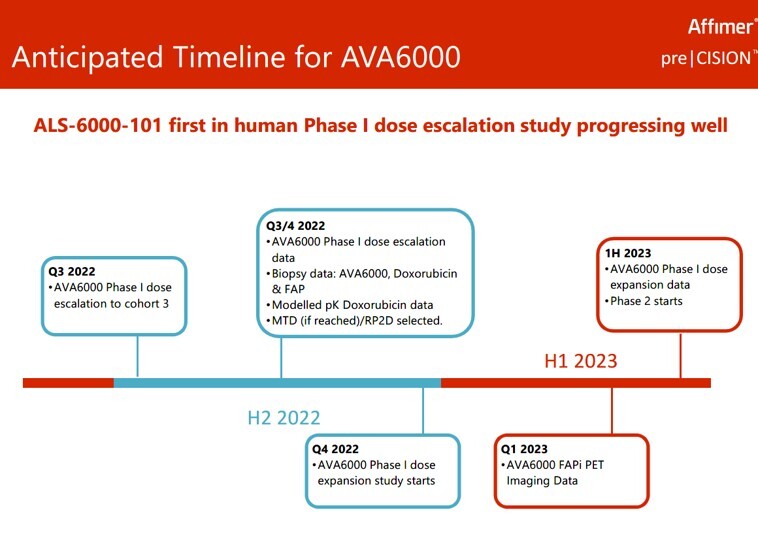
Meanwhile, I'll be keeping an eye on further trial data of which there's much more planned over the next 6 months.