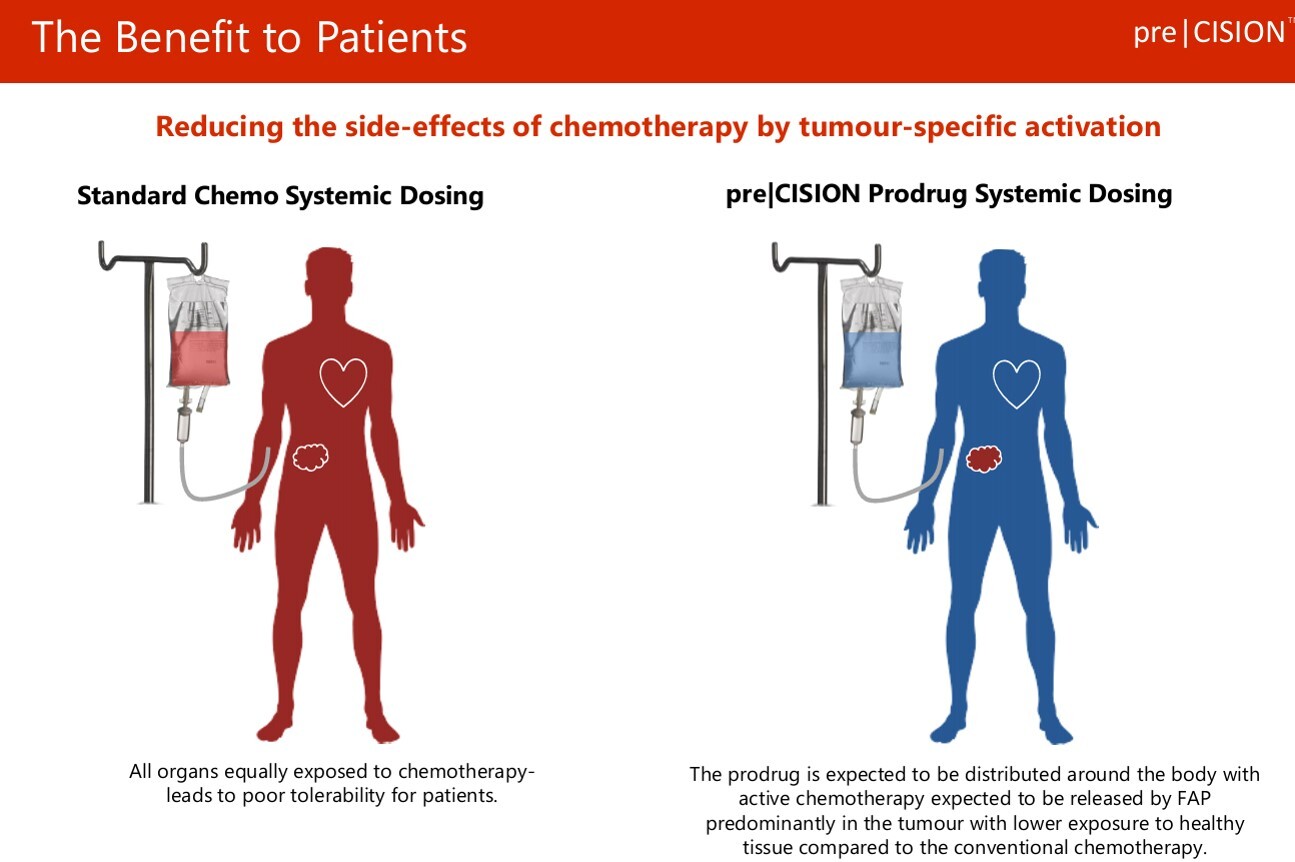
Doxorubicin is one of the most powerful chemotherapy drugs ever invented, & is used to treat a wide variety of cancers.
Historically though it’s been non-discriminatory. Not only killing cancerous cells, but also harming healthy tissue, especially around a patient’s heart. Hence dosing regimes have had to be limited for safety reasons - restricting efficacy & repeatability.
That is until potentially now.
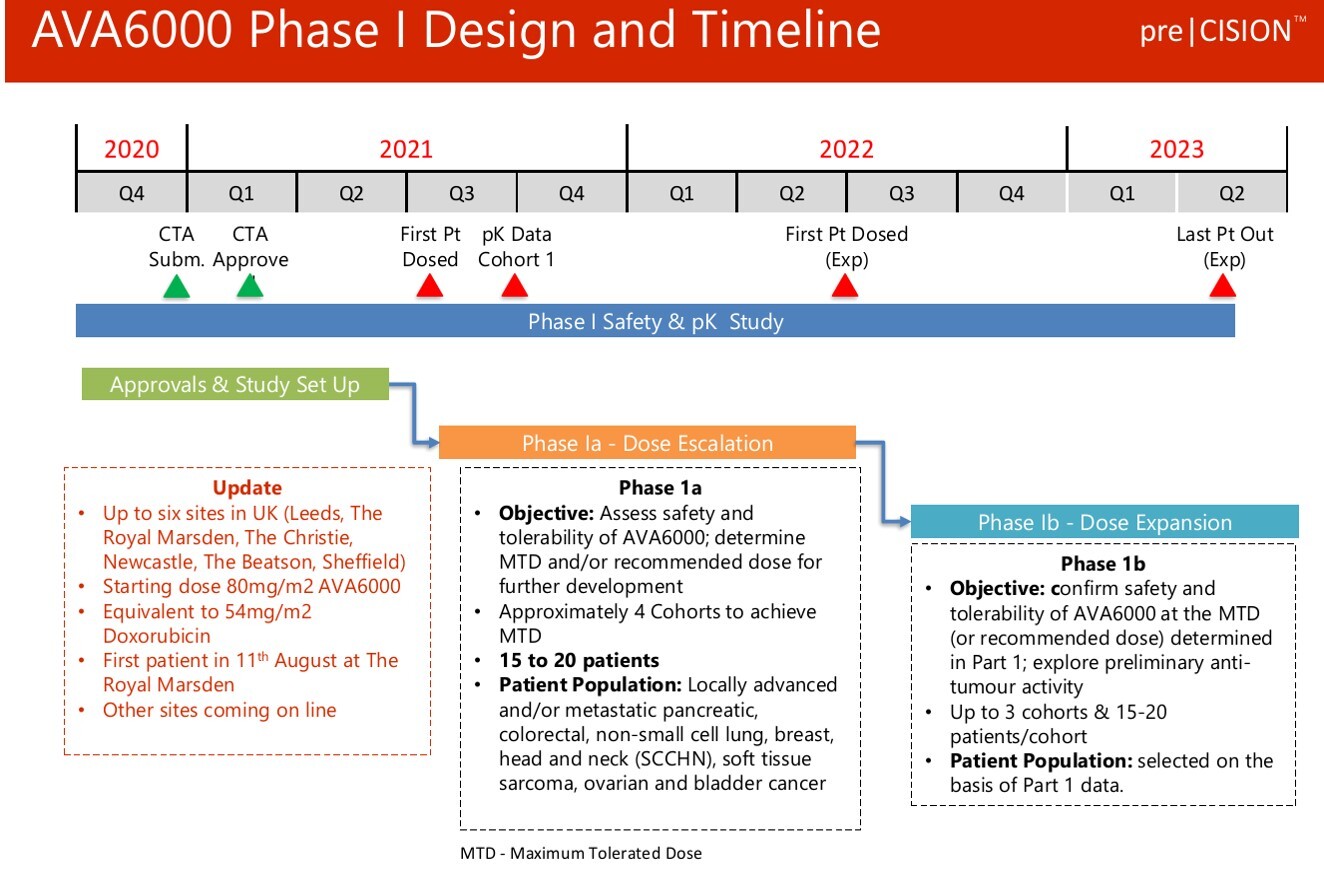
On 11th August,Avacta (AVCT ) commenced a Phase I multi-centre clinical trial evaluating its patented AVA6000 therapeutic – proDoxorubicin. With initial pharmacokinetic (PK) study data expected over the next month or so.
Additionally today came news that the FDA had also approved the testing of proDoxorubicin wrt a similar multi-centre Phase 1 clinical trial in the US - with patient enrollment scheduled for early 2022.
This is great news, especially since the UK study has now been progressing for >3 months. Suggesting to me, there’s been no major negative findings so far - as otherwise Avacta wouldn’t have progressed its FDA drug application.
CEO Alastair Smith, adding: “This is a major milestone. And provided that the study shows that the pre|CISION technology is effective in reducing systemic toxicity of Doxorubicin, then that would open up an extensive and proprietary pipeline for Avacta of next-generation chemotherapies with significant clinical and commercial advantages in a market that is expected to exceed $74bn by 2027”.