Another day, another major tick in the box for Avacta’s (AVCT) pre|CISION FAP-activated (fibroblast activation protein α) delivery platform.
The company announced this morning that its highly promising Phase I trial of AVA6000 Pro-doxorubicin "would advance to the 4th patient cohort using a higher 200mg/m2 dosage. After receiving a positive review of the safety and tolerability data from the 3rd cohort (at 160mg/m2 )."
This sounds like a significant step forward, since Doxorubicin is one of the most powerful chemotherapies ever invented. The only problem being that in its raw form, the drug kills both cancerous and healthy tissues indiscriminately, especially around the heart.
This means that, normally, the maximum approved dose is between 60-75mg/m2 as a monotherapy. So, given Avacta’s AVA6000 study is using very sick people, reporting such excellent safety data at higher concentration levels without onerous side affects is very encouraging indeed.
CEO Alastair Smith commented: " We are very much encouraged to move onto the 4th dose cohort in our ongoing Phase 1 dose escalation study. This very positive progress reflects the safety profile and tolerability demonstrated in patients enrolled in the study to date."
If ultimately successful, this could open up an enormous pipeline of next-generation pre|CISION pro-drug chemotherapies with significant clinical and commercial potential in a market that is expected to exceed $74bn by 2027. Within this, anthracyclines such as Doxorubicin are forecast to grow to $1.38bn by 2024.
Research house Trinity Delta has a 219p a share risk-adjusted intrinsic worth for the stock, with AVA6000 valued at 20p a share and the remaining pre|CISION platform at 103p.
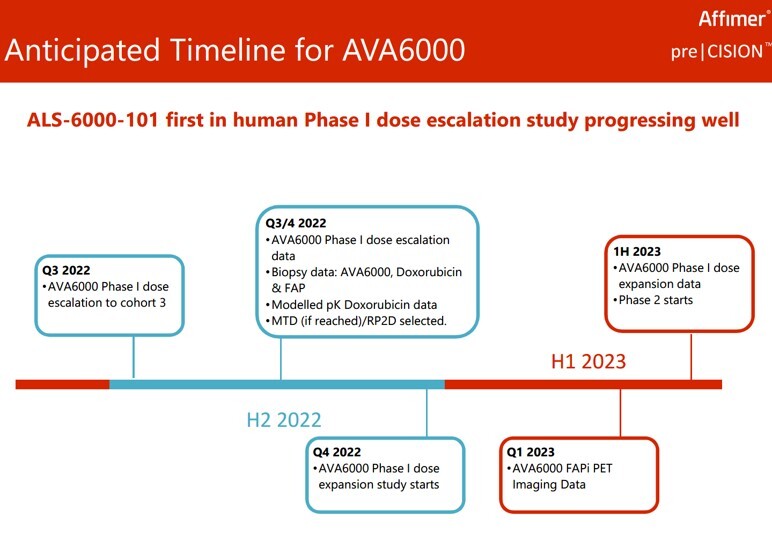
In terms of future newsflow, there’s plenty of additional data planned over the next 12 months (see chart) – not least biopsy and pK readings, alongside the important maximum tolerated dose decision in H2 2022. Or in other words, hopefully lots more to look forward to.